H202 Detection Strips & Biological Indicators


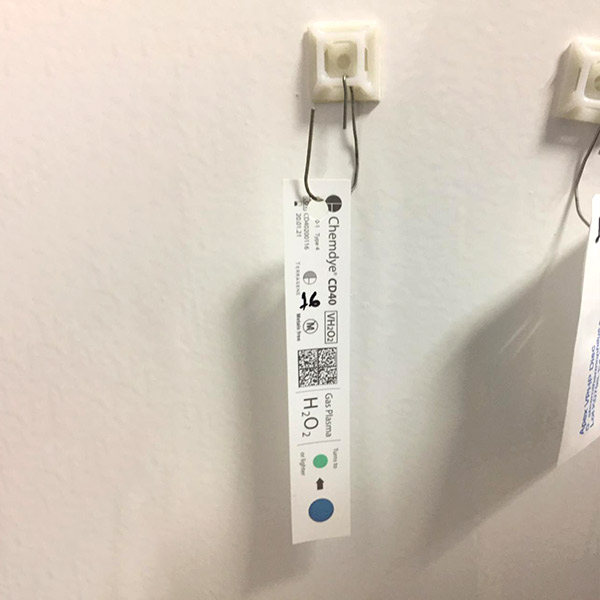
- Changes color when exposed to H2O2 fog during bio decontamination treatment.
- Helps in judging exposure of H2O2 fog across room space.
Biological Indicators are strong tools used to validate the efficacy
of sterilization and disinfection processes. Built with highly durable
spores, Biological Indicators provide a robust means of
ascertaining if a sterilization process has indeed eliminated
potential pathogens. Widely used in hospitals, pharmaceutical
production and laboratories, Biological Indicators validate
decontamination processes meet the stringent standards of
safety. By subjecting the sterilization cycle to a known population
of resistant microorganisms, Biological Indicators offer direct proof
of effectiveness for a system, enabling operators to validate
cleaning cycles and stay within industry guideliness.
These indicators are simple to install and read, delivering precise, actionable information to improve sterilization processes. They are resistant to diverse decontamination processes and are a requirement in those situations where the failure of sterilization, no matter how slight, may have devastating results. Biological Indicators' outcomes reinforce quality assurance programs, and they also provide an extra level of protection that is secondary to other disinfection protocols. Their incorporation into routine maintenance and validation procedures supplies ongoing monitoring of sterilization effectiveness, thus ensuring public health protection and maximum levels of environmental protection.
These indicators are simple to install and read, delivering precise, actionable information to improve sterilization processes. They are resistant to diverse decontamination processes and are a requirement in those situations where the failure of sterilization, no matter how slight, may have devastating results. Biological Indicators' outcomes reinforce quality assurance programs, and they also provide an extra level of protection that is secondary to other disinfection protocols. Their incorporation into routine maintenance and validation procedures supplies ongoing monitoring of sterilization effectiveness, thus ensuring public health protection and maximum levels of environmental protection.
Applications


Hospital


Pharmaceutical Plants

Food Processing

Laboratories


Animal House

Hatcheries


